High-Resolution Transcriptomic Profiling of the Heart During Chronic Stress Reveals Cellular Drivers of Cardiac Fibrosis and Hypertrophy
 Pinto Lab - Baker Heart and Diabetes Institute
Pinto Lab - Baker Heart and Diabetes Institute
Abstract
Background: Cardiac fibrosis is a key antecedent to many types of cardiac dysfunction including heart failure. Physiological factors leading to cardiac fibrosis have been recognized for decades. However, the specific cellular and molecular mediators that drive cardiac fibrosis, and the relative impact of disparate cell populations on cardiac fibrosis, remain unclear.
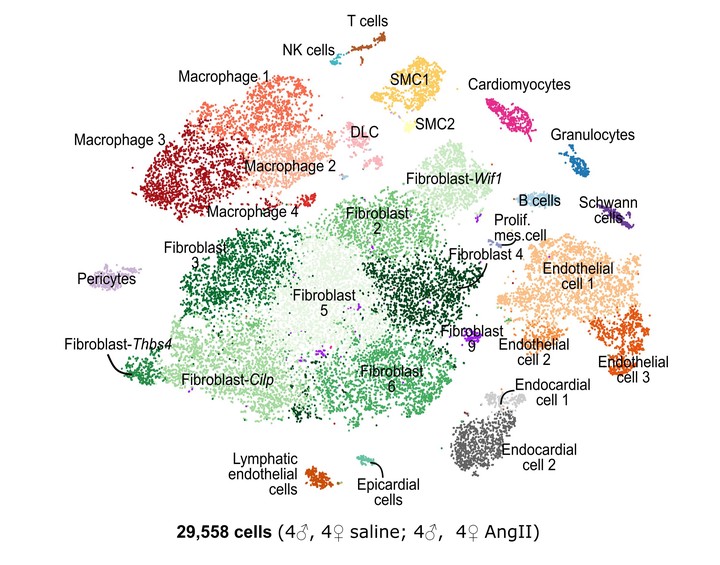
Methods: We developed a novel cardiac single-cell transcriptomics strategy to characterize the cardiac cellulome—the network of cells that forms the heart. This method was utilized to profile the cardiac cellular ecosystem in response to two weeks of continuous administration of Angiotensin II, a pro-fibrotic stimulus which drives pathological cardiac remodeling.
Results: Our analysis provides a comprehensive map of the cardiac cellular landscape uncovering multiple cell populations that contribute to pathological remodeling of the extracellular matrix of the heart. Two phenotypically distinct fibroblast populations—Fibroblast- Cilp and Fibroblast-Thbs4—emerged after induction of tissue stress to promote fibrosis in the absence of smooth muscle actin-expressing myofibroblasts, a key pro-fibrotic cell population. Following Angiotensin II treatment, Fibroblast-Cilp develops as the most abundant fibroblast subpopulation and the predominant fibrogenic cell type. Mapping intercellular communication networks within the heart, we identified key intercellular trophic relationships and shifts in cellular communication after Angiotensin II treatment, that promote development of a profibrotic cellular microenvironment. Further, the cellular responses to Angiotensin II and the relative abundance of fibrogenic cells were sexually dimorphic.
Conclusions: These results offer a valuable resource for exploring the cardiac cellular landscape in health and after chronic cardiovascular stress. These data provide insights into the cellular and molecular mechanisms that promote pathological remodeling of the mammalian heart, highlighting early transcriptional changes which precede chronic cardiac fibrosis.
Download
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||